 Can’t quite figure out how to calculate the amount of distilled water to add to 5&1/2 gal of raspberry wine with an acid of 1.3 to reduce it to .7 or .8, can you help me? Some place I thought you mentioned to use the pearson’s square.
Can’t quite figure out how to calculate the amount of distilled water to add to 5&1/2 gal of raspberry wine with an acid of 1.3 to reduce it to .7 or .8, can you help me? Some place I thought you mentioned to use the pearson’s square.
Thanks Knute
—–
Hello Knute,
I want to thank you for this question, and here’s why. The Pearson’s square is something that I’ve been meaning to write about for some time. It seems like every time I turn around I am referencing it in a blog post, but I have never done a blog post specifically on using the Pearson’s square to calculate adjustments to a wine.
And that’s a shame, because in my mind the Pearson’s square is one of the most underutilized calculating tools a winemaker has at their disposal. It can turn, what seems like, a very difficult math problem, and reduce it down to something visual that can be figured out in seconds. The Pearson’s square is the perfect tool for any wine blending scenario you can think of, including fortifying wines. It’s also ideal for calculating wholesale adjustments to the acidity or sugar content of a wine must.
What the Pearson square does is very simple.
It allows you to calculate how much of two liquids you will need to blend together (the ratio) to reach a specific target reading. In the case of wine making, how much of each of the two wines to blend together to reach a target reading of either acidity, specific gravity, alcohol… Anything that can be measured in the wine that has a linear or even scale can be targeted. The only requirements are that you know the reading of each of the two liquids to be blended and the target reading.
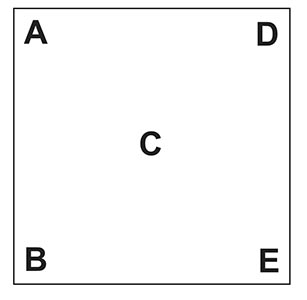 Here is the Pearson’s square. I am going to keep this as simple as possible:
Here is the Pearson’s square. I am going to keep this as simple as possible:
- A & B represent the reading of the two liquids to be blended.
- C is the target reading. The reading you wish to have.
- D & E represent how many parts of each liquid you need to blend (the ratio).
Knute, now lets take your situation as an example. You have a wine with a titratable acidity of 1.30%. By the way, this is a very high acid reading. I don’t imagine it taste very good. You want to know how much distilled water you need to add to the wine to bring it down to around .70% or .80%.
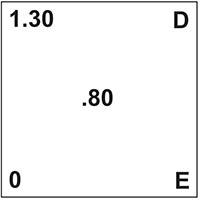 Here’s how this problem would set up on the Pearson’s square:
Here’s how this problem would set up on the Pearson’s square:
- A is the acidity level of your wine.
- B is the titratable acidity of water.
- C is the acidity level you would like to have.
- D & E is what we need to know. How much of each liquid to blend.
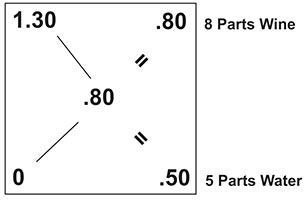 To solve for D & E you want to calculate the difference between A-C and the difference between B-C. In your case Knute, this would be the difference between 1.30 and .80 which comes out to .50. And, the difference between 0 and .80, which is .80. Here’s how this would play out on the Pearson’s square.
To solve for D & E you want to calculate the difference between A-C and the difference between B-C. In your case Knute, this would be the difference between 1.30 and .80 which comes out to .50. And, the difference between 0 and .80, which is .80. Here’s how this would play out on the Pearson’s square.
So what does this mean for your 5.5 gallons of raspberry wine?
It means that for every 8 parts of raspberry wine, you need to add 5 parts water. Or to put another way, to every 8 quarts of wine, you need to add 5 quarts of water.
You can also figure this down to ounces. There are 128 ounces in a gallon. You have 5.5 gallons of wine. That would be a total of 704 ounces. For every 8 ounces, you need to add 5 ounces of water.
If you take 704 ounce and divide it by 8, you will know how many 8 ounce portions of wine you have. It turns out you have 88 – 8 oz. portions in 5.5 gallons of wine. Now take the 88 portions and times it by the 5 ozs. of water you need for each portion. That’s how many ounce of water you need to add (5 x 88 = 440). This breaks down to:
- 3 Gallons (384 ozs.)
- 1 Quart (32 ozs.)
- 1 Pint (16 ozs.)
- 1 Cup (8 ozs.)
All of these add up to the 440 ounces. Add this much distilled water to the wine and it will have an acidity level of .80%.
 If you haven’t been able to tell yet, I like the Pearson’s square a lot. I find myself using it all the time. Not to take large swipes at a wine like Knute needed to, but to make minor adjustments. The Pearson’s square is almost a necessity when blending wines or when wanting to fortify a wine with distilled alcohol or brandy.
If you haven’t been able to tell yet, I like the Pearson’s square a lot. I find myself using it all the time. Not to take large swipes at a wine like Knute needed to, but to make minor adjustments. The Pearson’s square is almost a necessity when blending wines or when wanting to fortify a wine with distilled alcohol or brandy.
Happy Winemaking,
Ed Kraus
—–
Ed Kraus is a 3rd generation home brewer/winemaker and has been an owner of E. C. Kraus since 1999. He has been helping individuals make better wine and beer for over 25 years.

Thank you for the explanation of Preason Square. I have several places that it will come in handy, wine making, and also calculations when blending feeds for livestock.
Are you sure about using 0 as the pH of distilled water….? in the pearson’s square example
James, distilled water is not neutral as you are implying. It typically has a pH of about 6. In the example, we are talking about titratable acid in the distilled water, not pH. While there is some titratable acid in distilled water, it is small enough to just say 0%.
Is the distilled water ( using the Pearson’s square in Knute’s question) to be added at the beginning of fermentation or on a finished product with a high acid content ? It was called "wine" not "must", so am not certain. Thanks! Gloria
Gloria, you want to make any major adjustments to the acidity before the fermentation. So, add the water in the beginning.
Would not adding that much distilled water to a high acid must create a very lite or thin blend? Is this not what we use calcium carbonate to correct? Maybe this is referring to a concentrated raspberry juice?
Tony, it may have been a concentrate or a recipe that did require dilution with water. If there is an extreme case of high acidity you can use a combination of water dilution and acid reducing crystals. I would take a look at the article posted below with suggestions on how to lower acidity.
Getting A Handle On Wine Acidity
http://eckraus.com/wine-making-acidity/
thank you so much for this knowledge, i have struggled with this for the last three weeks till i came to see this Pearson square calculation, a more than grateful
Great knowledge…thanks.